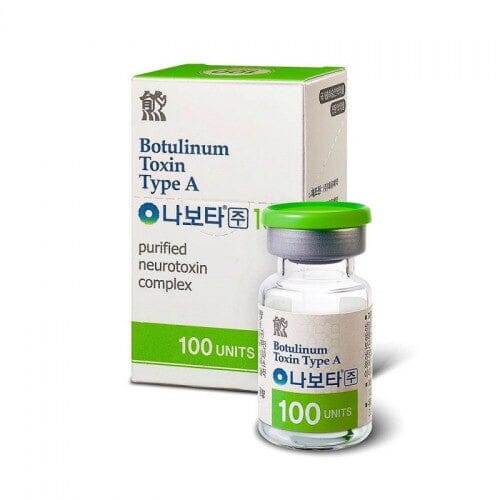
- Sakala tn 7-2 10141 Tallinn, Estonia
- (+372)-8111-9005
- info@lushfills.com
- Free Shipping above Є999.99
- MOQ: 5 Units

⚕️ lushfills – Licensed Professionals Only ⚕️
Our products are exclusively available to licensed healthcare professionals and registered healthcare businesses. These products must be used and administered ONLY by trained professionals to ensure safety, compliance, and proper application.
✅ Order Requirements:
• Proof of valid licensure is OBLIGATORY before order processing.
• Unauthorized purchases are STRICTLY PROHIBITED!. If you are not a licensed healthcare provider, DO NOT ORDER.
⚠️ Liability Disclaimer & 🔒 Regulatory Compliance:
We are not responsible for misuse, improper administration, or unauthorized use.To align and comply with the TOS and AUP of our Hosting Provider and E.U Good Distribution Practice (GDP) Guidelines, thorough License/Certificates verification MUST be done before we can process any order.